Abstract
Background: Iberdomide (IBER) is a potent cereblon E3 ligase modulator (CELMoD agent) with direct anti-tumor and immunomodulatory activities in multiple myeloma (MM). An ongoing phase 1b/2a multicenter, open-label, dose-escalation study has reported favorable efficacy and safety of IBER plus low-dose dexamethasone (DEX) in heavily pretreated patients with relapsed/refractory MM (RRMM). While previous studies focused on IMiD/CELMoD agent immune effects in peripheral blood (Amatangelo, ASH 2019), a large-scale characterization of pharmacodynamics in the bone marrow (BM) tumor microenvironment (TME) has not been reported. Here, we present data on BM aspirates (BMAs) of 99 individuals pre-/post-treatment with IBER in the largest study of TME immune dynamics of RRMM patients to date.
Methods: A mass cytometry (CyTOF) panel was designed/validated to capture deep and comprehensive immunophenotyping of T, B, and NK cell subpopulations. In all, staining with 37 metal-tagged Abs characterized 110 supervised cell phenotypes. Paired longitudinal assessment was conducted for viably preserved BMAs of IBER±DEX treated patients in dose-escalation of the CC220-MM-001 Ph 1b/2a study pre (SCREEN) and post treatment (cycle 2 day 15, C2D15). For a subset of patients (n=12), samples were available at disease progression (PD) . A training set analysis (n=64, data reported here) collected in North America, was validated against an independent test set (n=35) recruited in Europe. An R-based computational workflow and manual hierarchical gating identified subpopulations of B, T, NK and myeloid cells. In total, more than 5M immune cells were captured (approx. 40K per specimen). Cell populations are expressed as percentage of non-tumor bone marrow mononuclear cells (BMMC, i.e. CD45+CD66b-). P values are by Mann-Whitney U test for unpaired and Wilcoxon test for paired analyses.
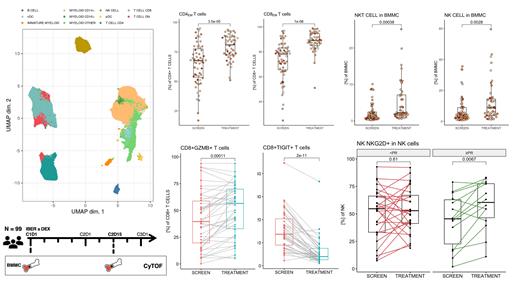
Results: IBER treatment resulted in profound immune shifts in the TME. B cells decreased (median SCREEN 3.3% vs C2D15 0.7%, p=0.001), with significant reduction of naïve (p=0.001) and regulatory B cells (p<0.001). Decrease occurred in CD4+ T cells (median 15.3% vs 8.9%, p<0.001), whereas CD8+ T cells increased (median 14.9% vs 17.4%). T cell subtypes showed a shift towards cytotoxic effector-memory phenotype (CCR7-CD45RA) (median 67.6% vs 80.9% of CD4+ T cells, p<0.001 and median 78.2% vs 89.2% of CD8+ T cells, p<0.001) with concurrent reduction of naive (CD45RA+CCR7+), central-memory (CD45RA-CCR7+) and EMRA (CD45RA+CCR7-) T cells. The shift towards a cytotoxic TME is confirmed by significant increases of GZMB+, HLA-DR+, ICOS+, Ki-67+, CXCR3+ and CD38+ activated CD8+ T cells (p≤0.001, paired). Conversely, CD8+ T cells expressing inhibitory checkpoints TIGIT and KLRG1 decreased significantly (p≤0.001, paired). Similar changes (with minor deviations) were noted in the CD4+ T cell compartment.
NK cells increased (median 4.2% vs 8.7%, p=0.003), with increase of both CD56hi cytokine-producing (median 1.9% vs 4.5%, p<0.001) and CD16+ cytolytic (median 1.8% vs 2.6%, p=0.06) subsets. NKG2A+ and NKG2D+ subsets were increased (p<0.001, paired), as well as subsets expressing markers of activation: GZMB, ICOS, CD38 and PD-1 (p<0.05, paired). TIGIT+ NK cells decreased significantly. NKT cells were also significantly increased (median 1.4% vs 2.2%, p<0.001). Increase of NK and NKT cells expressing the activating receptor NKG2D was limited to patients achieving best response of PR or better. Additional analyses correlating immune phenotypes at baseline/post-treatment with outcomes are ongoing and will be reported at the meeting, as will data on how prior treatment (e.g. with CD38-mAb) shapes the TME at baseline and affects response.
Conclusion: Our analysis on a large cohort of RRMM patients provides unique insights into the heterogeneity of the immune TME of heavily pretreated MM patients and how it changes upon treatment with IBER±DEX. We found significant increases of effector T and NK cells in paired analysis, demonstrating innate and adaptive immune enhancement in the MM bone marrow niche as an important mechanism of action. Importantly, these changes were observed throughout dose escalation and in patients previously refractory to lenalidomide/pomalidomide. The presented platform of large-scale immune profiling demonstrates a strategy to design and study rational combinations with (other) immune-enhancing therapies in MM.
Amatangelo: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Gooding: Bristol Myers Squibb: Research Funding. Jagannath: Legend Biotech: Consultancy; Bristol Myers Squibb: Consultancy; Karyopharm Therapeutics: Consultancy; Janssen Pharmaceuticals: Consultancy; Takeda: Consultancy; Sanofi: Consultancy. Pierceall: BMS: Current Employment, Current equity holder in publicly-traded company. Parekh: Foundation Medicine Inc: Consultancy; Amgen: Research Funding; PFIZER: Research Funding; CELGENE: Research Funding; Karyopharm Inv: Research Funding. Thakurta: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal